At The Royal Marsden I perform two roles: I'm a medical oncologist in gynecological cancers, but I also have an additional speciality in cancer genomics – the study of the genetic make-up of cancers. I carry out testing for inherited cancers and I also do a lot of work in molecular testing of tumours, to help inform treatment options for patients and work out a personalised programme for each of them. As clinical director of genomics at The Royal Marsden, I also oversee research programmes and innovation in molecular testing, early diagnosis and getting patients onto treatment as soon as possible.
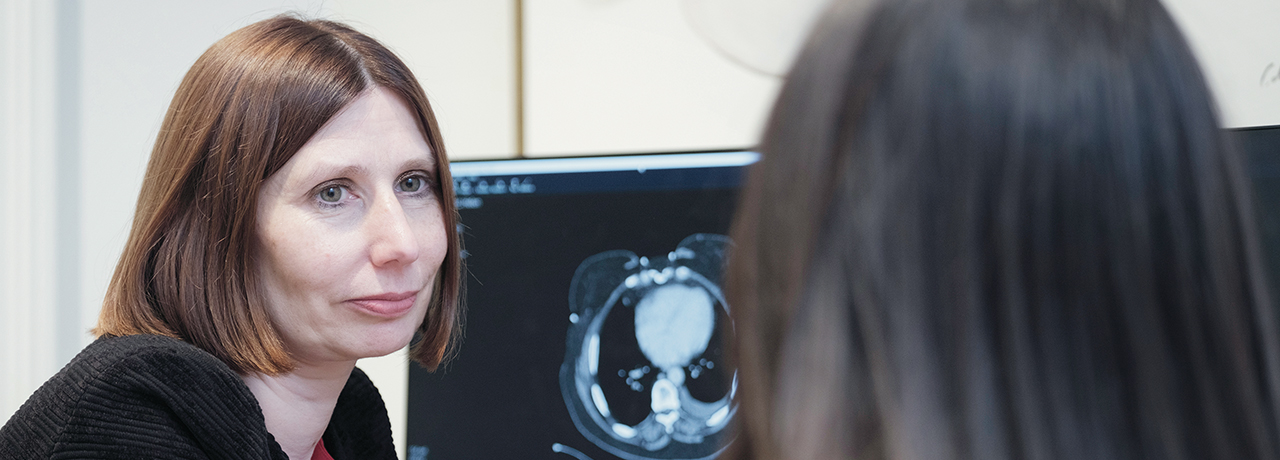
Genomic testing examines the DNA of cancer cells to find out what is making the cancer grow. Our genomics laboratories at The Royal Marsden are superb – we provide cancer diagnostic and genomic testing for a large part of the UK and a number of other countries, which send their samples to us for testing.
We’re currently the only laboratory in the UK that is accredited to carry out circulating tumour DNA (ctDNA) testing, also referred to as a liquid biopsy, which analyses DNA fragments released by cancer cells into the bloodstream. This involves taking a blood sample, rather than having to perform a biopsy of a tumour site, which is a lot more pleasant for the patient. We have also developed our own in-house RNA-DNA panels, Ribonucleic acid (abbreviated as RNA) is a nucleic acid present in all living cells that has structural similarities to DNA. These panels are very comprehensive, so if any new genes become relevant for a tumour type that haven’t previously been assessed, we can update our information very quickly.
In oncology today, we’re moving from treatment based on the type of cancer that a patient has to a much more personalised service, where we consider specific information from a patient’s tumour to decide the best treatment for them. In a very short time, we’ve reached a point where there’s almost no type of cancer in which genomics doesn’t play a role, whether that’s in accurate diagnosis, informing prognosis or selecting the most appropriate treatment with the least chance of toxicity for the patient.
To give patients the maximum benefit from treatment, we locate the mutations that have caused the cancer and affect how it behaves. We look for a way to block these – it’s like looking for the switch that turned the cancer on and trying to turn it back off again.
If you look at the progress we’ve made in cancer treatment over the past few years, it’s all about finding targeted treatments. A really good example of this is in lung cancer. Previously, everyone with advanced lung cancer received chemotherapy, which often helped with the symptoms, but not with extending lives – as people usually died within two years of their diagnosis. These days, we sequence tumours – in other words, analyse their DNA – in patients thought to have lung cancer, and more than half will have a mutation in the tumour that we can target with a specific drug.
The difference we are now seeing is that patients with advanced cancer, who previously might have survived another six or 12 months, are still alive more than 10 years later and living quite normal lives during that time. The ability to find and target the driver of the cancer makes a fundamental difference to patient outcomes.

The genomics can be divided into two parts. There are the changes that are just found in the tumour – we call these somatic changes. Then there are those that are inherited – one in six cancers has an inherited cause. These are important to find because many of the newer drugs are specifically aimed at those with inherited forms of cancer.
Finding inherited forms of cancer also presents the opportunity for prevention in family members – if we can find relatives carrying the same gene mutation then we can prevent them from developing the same cancer using risk-reducing interventions or appropriate screening. As most of these genes can cause more than one type of cancer, we also have the chance to intervene and prevent other cancers from occurring.
My second specialty involves gynaecological cancers, and I frequently see patients with these conditions to discuss necessary tumour testing, including visitors from overseas who haven’t had such tests routinely. Decisions on the type of treatment required – such as chemotherapy or immunotherapy – increasingly rely on these tests.
Patients often visit us from abroad because such tests are not offered locally. Occasionally there are treatments that aren’t available in other countries that potentially can be started with us at The Royal Marsden, and we can then work with the patient’s local team to continue the treatment when they return home.
Looking ahead, we are exploring earlier detection of returning cancers in those who have completed treatment. At present, such patients have lots of follow-up scans to make sure that their cancer isn’t returning.
In patients where breast cancer has come back, for example, it takes 10 to 12 months before we can detect this on a scan. We are looking at various tumour types using liquid biopsies to try and detect very small amounts of tumour cells in the blood much sooner.
I think we’ll be looking at how we can use this more widely in the future to detect cancer at very early stages. This will also be much easier for patients, who will be able to have a blood test every six months, rather than the inconvenience of regular scans.
We are also investigating how we can use blood tests to monitor people considered at high risk of developing cancer and pick up any issues at an early stage, giving us the best chance of curing them. For the types of tumours that currently don’t have many treatment options we are working with pharmaceutical companies to develop more effective drug treatments.
I run a number of clinical trials exploring molecular sequencing and helping to determine the best treatments for patients on the basis of their molecular changes. I’m also running a large study looking at patients with endometriosis, which is an increasing risk factor in a number of cancers, particularly ovarian and endometrial cancer. We’d like to be able to predict endometriosis patients who are at risk of developing cancer.
In addition, I’m involved in setting up screening programmes for patients with inherited gene syndromes, looking for the most effective ways to detect cancer at an early stage. The key is to identify problems as soon as possible, to give the best chance of successful treatment.
For more information about The Royal Marsden Private Care or to refer a patient, please:
Visit: www.royalmarsden.nhs.uk/private
Email: int@rmh.nhs.uk
Call: +44 (0)20 7808 2063